Linking Complaints, Audits, and Deviations in a Centralized CAPA Workflow
The Strategic Need for CAPA Software for Medical Device Industry
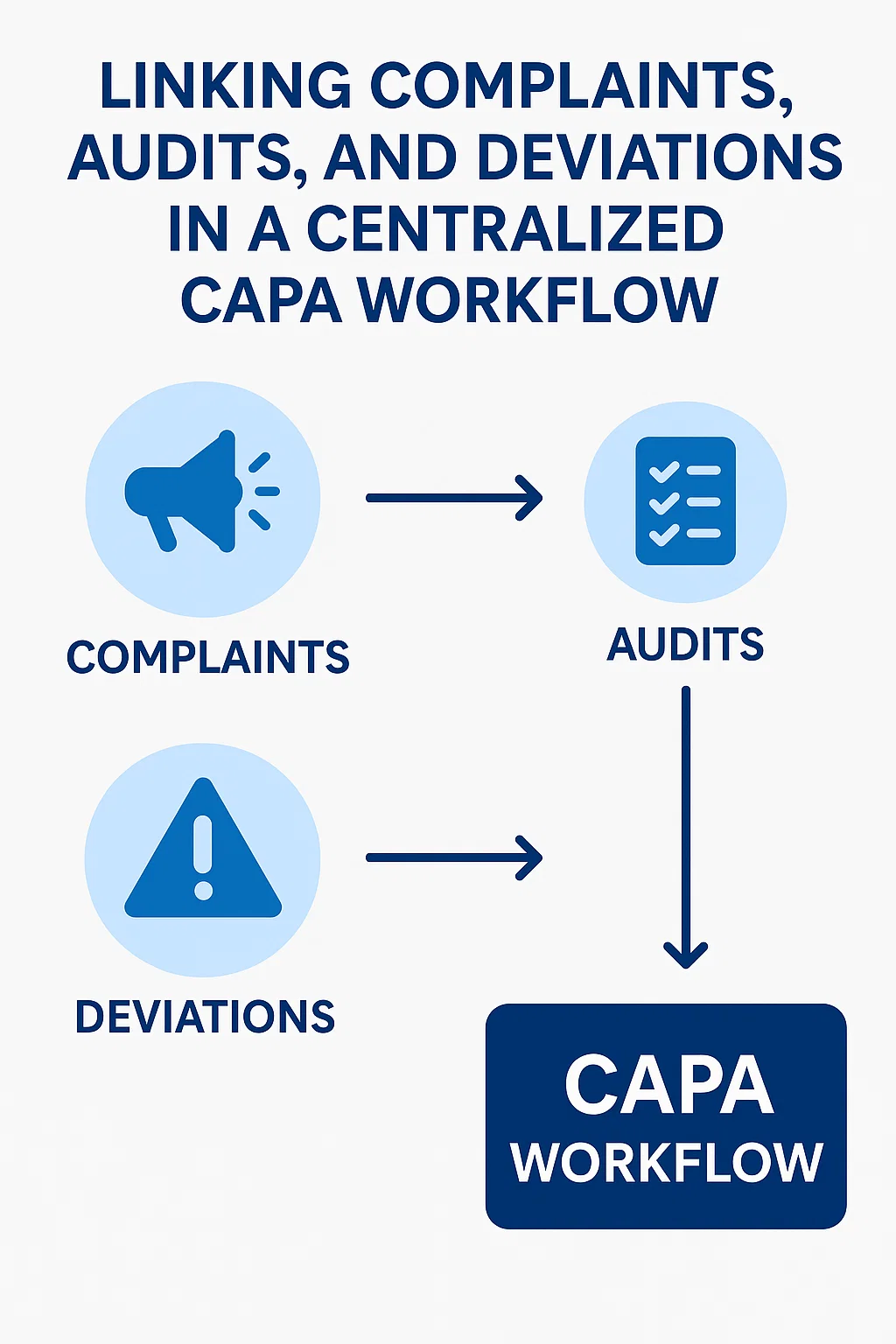
In the current regulatory climate, medical device manufacturers are increasingly investing in advanced CAPA Software for Medical Device Industry use to maintain control over quality and compliance. A centralized CAPA framework connects the dots between complaints, internal audits, and deviations, transforming disconnected quality events into cohesive, traceable workflows. Such integration is essential for ensuring regulatory alignment and achieving business-wide transparency, especially under frameworks such as FDA 21 CFR Part 820 and ISO 13485.
By linking these quality events within a unified system, organizations can gain real-time insights, ensure corrective actions are not isolated, and support ongoing compliance efforts. This helps mitigate systemic risks while supporting the overarching quality management strategy.
Integrating Complaints into a Closed-Loop CAPA Workflow
Complaints from customers, healthcare providers, and end-users are critical signals that can highlight deeper systemic issues. CAPA software for medical device industry enables manufacturers to automatically funnel complaints into the CAPA lifecycle, allowing early detection of recurring quality issues and failure modes.
The ability to capture, assess, and triage complaints in real-time ensures that organizations can prioritize high-risk incidents and initiate root cause analysis faster. An integrated quality management system streamlines this process, reducing manual handoffs and ensuring traceability from initial complaint to final resolution.
Using Audits to Trigger Preventive and Corrective CAPAs
Audits—both internal and external—often uncover process inefficiencies, documentation gaps, or noncompliances that demand a prompt response. A modern CAPA software for medical device industry provides automated links between audit findings and the CAPA process. Whether it's a supplier audit, regulatory inspection, or internal assessment, findings can be logged and routed to appropriate stakeholders within the centralized CAPA workflow.
This immediate connection between audit data and the CAPA system ensures no observation is left unaddressed. Organizations can also track audit effectiveness and closure rates over time, offering valuable insights for continuous improvement initiatives and long-term compliance planning.
Connecting Deviations to CAPAs for Root Cause Analysis
Process deviations—unexpected departures from defined procedures—are often early warning signs of quality drift. CAPA software for medical device industry offers robust functionality to log, assess, and escalate deviations. Once a deviation is logged, it can be immediately linked to an existing or new CAPA record based on severity and impact analysis.
Centralizing these events enables quality leaders to observe patterns across different product lines or manufacturing facilities. This enhances visibility and creates a foundation for advanced analytics that predict future deviations and automate preventive actions.
Supporting Quality Management Objectives Through CAPA Integration
Quality management in medical device manufacturing hinges on proactive detection and resolution of quality events. A centralized CAPA platform allows integration across various subsystems—complaints, audits, and deviations—enabling a comprehensive view of operational risk.
Through role-based dashboards, cross-functional teams can collaborate in real time, aligning CAPA management with overarching quality goals. This integration elevates the efficiency of the quality management system, enhancing compliance, product reliability, and customer satisfaction.
Automating Workflows for Faster CAPA Resolutions
One of the main advantages of modern CAPA software for medical device industry applications is workflow automation. Instead of relying on fragmented manual tracking methods, CAPA workflows are digitized and governed by pre-configured rules. This ensures consistent review, approval, and closure across all quality events.
Automated routing of CAPA tasks ensures accountability at every stage. Notifications, reminders, and escalation logic keep the process on track, reducing cycle times and enhancing responsiveness to regulatory scrutiny. This is particularly beneficial in global operations where alignment across geographies is critical.
Enhancing Traceability and Compliance Through Digital Linkages
Linking complaints, audits, and deviations in a centralized system improves traceability, which is a cornerstone of regulatory compliance. CAPA software for medical device industry platforms maintain a complete audit trail of all actions taken, approvals made, and documentation updates.
This level of traceability is critical for demonstrating compliance with regulatory standards such as ISO 13485 and FDA requirements. It allows quality teams to quickly respond to inspection requests and provides a structured foundation for compliance reporting and management review activities.
Driving a Culture of Accountability in CAPA Management
A well-integrated CAPA framework doesn't just enhance processes; it fosters a culture of ownership and accountability. By linking quality events to root cause investigations and action plans, every department is aware of its role in maintaining product integrity and compliance.
CAPA software encourages transparency across departments by providing visibility into issue status, resolution timelines, and trends in recurring Nonconformances. This leads to a stronger quality culture that extends beyond the QA/RA teams to include R&D, manufacturing, and supply chain functions.
Leveraging Analytics to Strengthen CAPA Strategies
Modern CAPA platforms offer analytics and reporting dashboards that help stakeholders understand the effectiveness of their CAPA processes. With built-in analytics, quality leaders can measure CAPA resolution times, recurring issue frequency, and root cause distribution.
These insights help inform strategic quality decisions and can uncover latent trends. Whether the issue stems from design, supplier performance, or production execution, analytics provide actionable data that support better decision-making.
Conclusion: Why ComplianceQuest is Essential in 2025
As regulatory expectations intensify and global supply chains become more complex, a connected CAPA framework is no longer optional—it’s a business imperative. ComplianceQuest offers a cloud-native, AI-enabled platform designed to address the multifaceted needs of the modern medical device industry.
With modules that integrate complaints, audits, deviations, and CAPAs into one seamless system, ComplianceQuest ensures quality management is proactive, not reactive. In 2025, organizations that adopt ComplianceQuest will be better positioned to reduce risk, enhance patient safety, and accelerate market readiness while staying fully aligned with FDA and ISO expectations. It is the definitive solution for medical device companies seeking operational excellence and enduring compliance through a modern digital quality ecosystem.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- الألعاب
- Gardening
- Health
- الرئيسية
- Literature
- Music
- Networking
- أخرى
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- Script
- App
